glucose
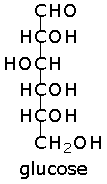
Glucose is an aldohexose - the natural form, D-glucose is also called dextrose. Glucose is biologically important, and is one of the products of photosynthesis by plants and some prokaryotes. Glucose is an important precursor in the synthesis of glycogen (animals), polysaccharides, lactose, cellulose and starch (plants), proteins and lipids, and vitamin C (plants, most animals).
Glucose is an important fuel in energy yielding catabolic pathways such as glycolysis, the pentose phosphate pathway, and the citric acid cycle. In the catabolic pathway gluconeogenesis, animals convert non-carbohydrate intermediates such as pyruvate and glycerol into glucose. Animals and fungi convert glycogen to glucose (glycogenolysis), while plants hydrolyse the storage form starch into glucose.
Glycosylation involves the addition of saccharides to proteins and lipids, and is one of four principal co-translational and post-translational modification steps in the synthesis of membranous and secretory proteins. Most proteins synthesized in the rough ER undergo glycosylation, which is an enzyme-directed site-specific process, as opposed to the non-enzymatic chemical reaction of glycation (below). There are two types of glycosylation: N-linked glycosylation to the amide nitrogen of asparagine side chains and O-linked glycosylation to the hydroxy oxygen of serine and threonine side chains.
Glucose can form formaldehyde under abiotic conditions, a propery that probably was utilized by primitive biochemical systems. A more important property of glucose to advanced metabolic forms is the low tendency of glucose, in comparison to other hexose sugars, to react non-specifically with the amino groups of proteins. This glycation reaction reduces or destroys the function of many enzymes. Glucose's low rate of glycation is due to the tendency of glucose to adopt the less reactive cyclic isomeric form (below). Nevertheless, many of the long-term complications of diabetes, such as blindness, kidney failure, and peripheral neuropathy probably result from the glycation of proteins or lipids.
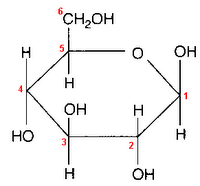
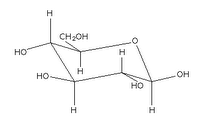
Glucose can take up a linear or a cyclic form. The ring form results from bonding between the aldehyde C atom and the C-5 hydroxyl group, creating an intramolecular hemiacetal. By virtue of the ring form's resemblance to pyran, glucose is also termed glucopyranose.
Glucose has 4 optic centers, so glucose has (4²-1) = 15 possible optical stereoisomers, of which only 7 occur biologically. Of these galactose (Gal) and mannose (Man) are the most important. The eight isomers (including glucose) are all diastereoisomers in relation to each other and all belong to the D-series.
An additional asymmetric center at C-1 (called the anomeric carbon atom) is created when glucose cyclizes, so two ring structures, called anomers, α-glucose and β-glucose. The α-and β- forms differ structurally in the orientation of the hydroxyl group linked to C-1 in the ring. The designation α means that the C-1 hydroxyl group is below the plane of the ring in the Haworth projuection, while the β means it is above the ring. The α and β forms slowly interconvert in aqueous solution in a process called mutarotation solution to a final stable ratio of α:β = 36:64.
Image of glucose representations from Fisher to Haworth.
Animated image of D-Glucose shifting into β-D-glucose.








































0 Comments:
Post a Comment
<< Home