Cofactors, Coenzymes, biosynthetic intermediates
In general, a cofactor is any substance required to cooperate with an enzyme that catalyzes a specific reaction. Cofactors are non-proteinaceous substances that assist enzymes in performing catalytic actions. Cofactors may be cations (metal ions) or organic molecules known as coenzymes (vitamins).
Cofactors may be altered temporarily during the reaction which they assist, but they return to their original state after participating in catalysis, so they are not ultimately changed by the reaction.
Cofactors may be loosely or tightly bound to the enzyme. Organic, loosely bound cofactors are called coenzymes, and play an accessory role in enzyme-catalyzed processes, often by acting as a donor or acceptor of a substance involved in the reaction. When combined with an inactive apoenzyme, coenzymes form a complete, active enzyme called the holoenzyme. ATP and NAD+ are common coenzymes, and are loosely bound to the enzyme. Many coenzymes are phosphorylated water-soluble vitamins. Heme is tightly, covalently bound, and as such is a prosthetic group.
Prosthetic groups differ from cofactors in being tightly (often covalently) bound to a particular enzyme molecule. Haem is an example of a prosthetic group found in cytochromes and haemoglobin, which carries electrons and/or oxygen.
In molecular genetics, cofactors are proteins that interact with transcription machinery, transducing regulatory information between core RNA polymerase machinery and gene-specific transcription factors. 'Mediator' is the most universal cofactor known today – it is a modular complex serving as the interface between gene-specific RNA pol II machinery. Mediator is also needed for response to other regulatory proteins such as activators and repressors of transcription.
Many molecules are important intermediates in biochemical pathways.
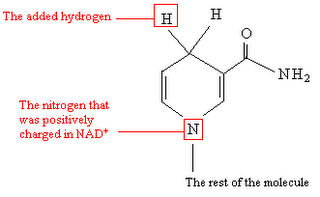
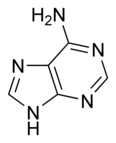
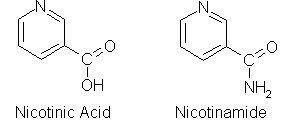
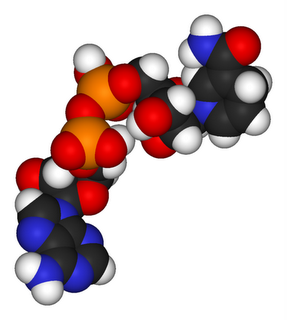
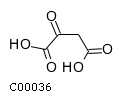
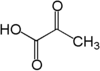
• acetyl-CoA • NADH : NAD+ : NADPH : NADP+ : Nicotinamide adenine dinucleotide : • pyruvate • • •
Cofactors may be altered temporarily during the reaction which they assist, but they return to their original state after participating in catalysis, so they are not ultimately changed by the reaction.
Cofactors may be loosely or tightly bound to the enzyme. Organic, loosely bound cofactors are called coenzymes, and play an accessory role in enzyme-catalyzed processes, often by acting as a donor or acceptor of a substance involved in the reaction. When combined with an inactive apoenzyme, coenzymes form a complete, active enzyme called the holoenzyme. ATP and NAD+ are common coenzymes, and are loosely bound to the enzyme. Many coenzymes are phosphorylated water-soluble vitamins. Heme is tightly, covalently bound, and as such is a prosthetic group.
Prosthetic groups differ from cofactors in being tightly (often covalently) bound to a particular enzyme molecule. Haem is an example of a prosthetic group found in cytochromes and haemoglobin, which carries electrons and/or oxygen.
In molecular genetics, cofactors are proteins that interact with transcription machinery, transducing regulatory information between core RNA polymerase machinery and gene-specific transcription factors. 'Mediator' is the most universal cofactor known today – it is a modular complex serving as the interface between gene-specific RNA pol II machinery. Mediator is also needed for response to other regulatory proteins such as activators and repressors of transcription.
Many molecules are important intermediates in biochemical pathways.
• acetyl-CoA • NADH : NAD+ : NADPH : NADP+ : Nicotinamide adenine dinucleotide : • pyruvate • • •

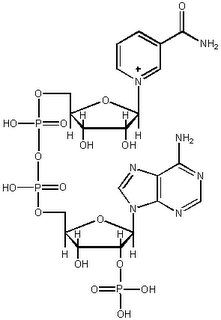
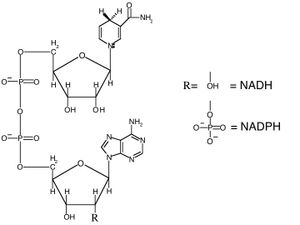 Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide